
2002, 23, 555–558.O_n + nO_2\]Īs noted here, the formulas of many carbohydrates can be written as carbon hydrates, \(C_n(H_2O)_n\), hence their name. Knoevenagel condensation over acidic zeolite. Isomerization of D-glucose to D-fructose over metallosilicate solid bases. Glucose-fructose isomerisation promoted by basic hybrid catalysts. Chiral evolution of carbon dots and the tuning of laccase activity. Chiral carbon dots-based nanosensors for Sn(II) detection and lysine enantiomers recognition. Hybrid supraparticles of carbon dots/porphyrin for multifunctional tongue-mimic sensors. Chirality transfer from graphene quantum dots. Highly fluorescent chiral N-S-doped carbon dots from cysteine: Affecting cellular energy metabolism. Revealing the nature of optical activity in carbon dots produced from different chiral precursor molecules. Metal-chelated polymer nanodiscs for NMR studies. Degradation study of biobased polyester-polyurethane and its nanocomposite under natural soil burial, UV radiation and hydrolytic-salt water circumstances. Environmentally friendly polyurethane dispersion derived from dimer acid and citric acid. Nuclear magnetic resonance reveals molecular species in carbon nanodot samples disclosing flaws. Resolution of infrared spectra and kinetic analysis of mutarotation of D-glucose in water by sequential rank analysis. Manganese oxide as an alternative to vanadium-based catalysts for effective conversion of glucose to formic acid in water. Kilogram-scale synthesis and functionalization of carbon dots for superior electrochemical potassium storage. Carbon quantum dots and their derivative 3D porous carbon frameworks for sodium-ion batteries with ultralong cycle life. Maltase decorated by chiral carbon dots with inhibited enzyme activity for glucose level control. Chiral control of carbon dots via surface modification for tuning the enzymatic activity of glucose oxidase. Rational design of multicolor-emitting chiral carbonized polymer dots for full-color and white circularly polarized luminescence. Transfer of axial chirality to the nanoscale endows carbon nanodots with circularly polarized luminescence. Natural deep eutectic solvent assisted synthesis and applications of chiral carbon dots. Chiral carbon dots derived from serine with well-defined structure and enantioselective catalytic activity. Aptamer-modified Cu 2+-functionalized C-dots: Versatile means to improve nanozyme activities-“Aptananozymes”. One-step hydrothermal synthesis of chiral carbon dots with high asymmetric catalytic activity for an enantioselective direct aldol reaction. Mapping the surface groups of amine-rich carbon dots enables covalent catalysis in aqueous media. Recent advances in chiral carbonized polymer dots: From synthesis and properties to applications. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Opportunities and challenges for combining chemo- and biocatalysis. Making chiral salen complexes work with organocatalysts. Facile access to chiral non-natural amino acids.
Chiral diene ligands in asymmetric catalysis. Advances in chiral metal-organic and covalent organic frameworks for asymmetric catalysis. Enantioselective behavior of environmental chiral pollutants: A comprehensive review.
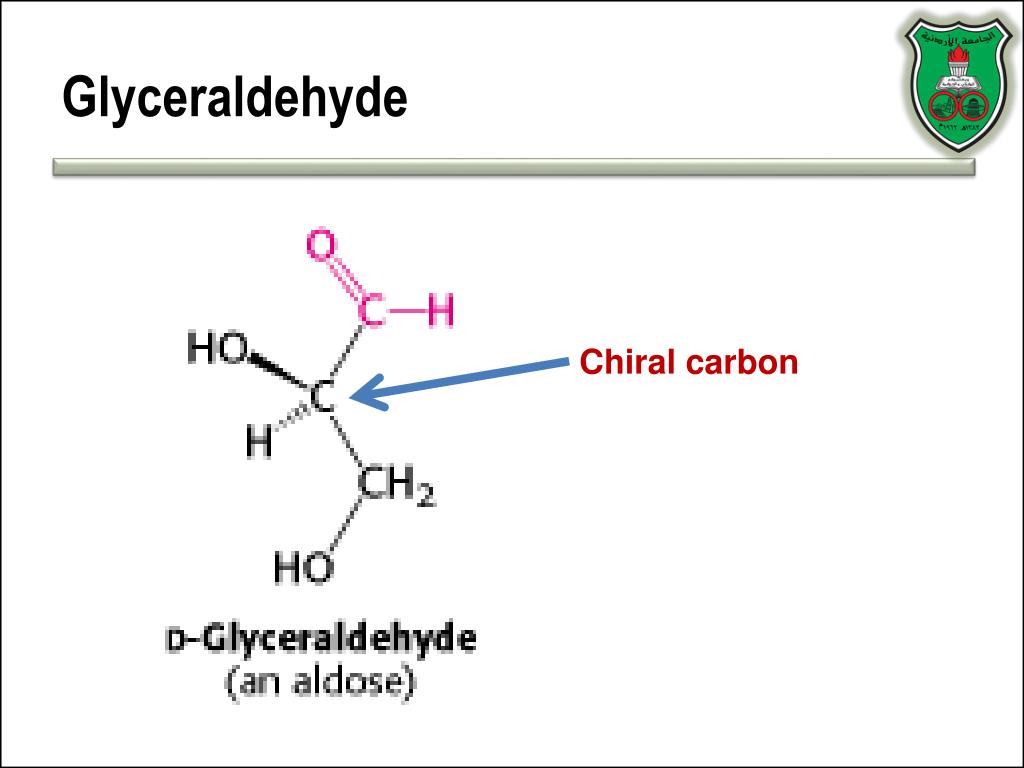
CHIRAL CARBON IN GLUCOSE SERIES
The need to change the approach to the safe use of herbicides by developing chiral and environmentally friendly formulations: A series of enantioselective (R)- and (S)-phenylethylammonium chloroacetates.

Theoretical calculations combined with various contrast experiments (temperature and pH) demonstrate that the selectively electrocatalytic capacity of chiral CDs toward Trp isomers is due to the different hydrogen-bond interactions between chiral CDs and Trp. L type of CDs (LCDs) is more likely to catalyze L-tryptophan (Trp) than D-Trp with the selective factor (/ L// D) of 1.60, whereas the D type of CDs (DCDs) tends to catalyze D-Trp (/ L// D: 0.63). These chiral CDs have been demonstrated that they have selective capacity for electrocatalytic oxidization of tryptophan enantiomers. The formation of chiral CDs involves the processes of isomerization and aldol condensation. Herein, we report a facile one step base-catalyzed aldol condensation to fabricate the chiral CDs from glucose at ambient temperature and pressure. Chiral carbon dots (CDs) as carbon-based chiral catalysts show great potential in chiral catalysis. Chiral catalysis is one of the most direct and effective approach to obtain pure optical enantiomers.


 0 kommentar(er)
0 kommentar(er)
